- Thyroid
- Beyond Acute COVID-19: Investigating the Incidence of Subacute Thyroiditis in Long COVID-19 in Korea
-
Jeongmin Lee, Gi Hyeon Seo, Keeho Song
-
Endocrinol Metab. 2023;38(4):455-461. Published online August 8, 2023
-
DOI: https://doi.org/10.3803/EnM.2023.1711
-
-
2,689
View
-
182
Download
-
3
Web of Science
-
4
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
The correlation between acute coronavirus disease 2019 (COVID-19) and subacute thyroiditis (SAT) has not been clearly investigated in “long COVID” patients. We aimed to investigate the incidence of SAT during convalescence and after the acute phase of COVID-19, comparing with that of the general population.
Methods
Data from a total of 422,779 COVID-19 patients and a control group of 2,113,895 individuals were analyzed. The index date was defined as the date 3 months after confirmation of COVID-19. The incidence rate (IR) of SAT and hazard ratios (HRs) were calculated per 100,000 persons. Subgroup analysis included analysis of HRs 90–179 and 180 days post-COVID-19 diagnosis; and additional analysis was conducted according to hospitalization status, sex, and age group.
Results
The IR of SAT was 17.28 per 100,000 persons (95% confidence interval [CI], 12.56 to 23.20) in the COVID-19 group and 8.63 (95% CI, 6.37 to 11.45) in the control group. The HR of COVID-19 patients was 1.76 (95% CI, 1.01 to 3.06; P=0.045). The HR of SAT was 1.39 (95% CI, 0.82 to 2.34; P=0.220) up to 6 months after the index date and 2.30 (95% CI, 1.60 to 3.30; P<0.001) beyond 6 months. The HR for SAT among COVID-19 patients was 2.00 (95% CI, 1.41 to 2.83) in hospitalized patients and 1.76 (95% CI, 1.01 to 3.06) in non-hospitalized patients compared to the control group. The IR of SAT was 27.09 (95% CI, 20.04 to 35.82) for females and 6.47 (95% CI, 3.34 to 11.30) for males. In the 19 to 64 age group, the IR of SAT was 18.19 (95% CI, 13.70 to 23.67), while the IR was 9.18 (95% CI, 7.72 to 10.84) in the 65 to 69 age group.
Conclusion
SAT could be a potential long-term complication of COVID-19. Long-term surveillance for thyroid dysfunction is needed especially in hospitalized, female and young-aged subjects.
-
Citations
Citations to this article as recorded by  - Thyroid dysfunction in COVID-19
David Tak Wai Lui, Chi Ho Lee, Yu Cho Woo, Ivan Fan Ngai Hung, Karen Siu Ling Lam
Nature Reviews Endocrinology.2024;[Epub] CrossRef - Subacute Thyroiditis in the Time of COVID-19
Hwa Young Ahn
Endocrinology and Metabolism.2024; 39(1): 186. CrossRef - Occult endocrine disorders newly diagnosed in patients with post-COVID-19 symptoms
Yasuhiro Nakano, Naruhiko Sunada, Kazuki Tokumasu, Hiroyuki Honda, Yuki Otsuka, Yasue Sakurada, Yui Matsuda, Toru Hasegawa, Daisuke Omura, Kanako Ochi, Miho Yasuda, Hideharu Hagiya, Keigo Ueda, Fumio Otsuka
Scientific Reports.2024;[Epub] CrossRef - rRisk of incident thyroid dysfunction in the post-acute phase of COVID-19: a population-based cohort study in Hong Kong
David Tak Wai Lui, Xi Xiong, Ching‐Lung Cheung, Francisco Tsz Tsun Lai, Xue Li, Eric Yuk Fai Wan, Celine Sze Ling Chui, Esther Wai Yin Chan, Franco Wing Tak Cheng, Lanlan Li, Matthew Shing Hin Chung, Chi Ho Lee, Yu Cho Woo, Kathryn Choon Beng Tan, Carlos
Endocrine Practice.2024;[Epub] CrossRef
- Diabetes, obesity and metabolism
- Efficacy of Gemigliptin Add-on to Dapagliflozin and Metformin in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Study (SOLUTION)
-
Byung Wan Lee, KyungWan Min, Eun-Gyoung Hong, Bon Jeong Ku, Jun Goo Kang, Suk Chon, Won-Young Lee, Mi Kyoung Park, Jae Hyeon Kim, Sang Yong Kim, Keeho Song, Soon Jib Yoo
-
Endocrinol Metab. 2023;38(3):328-337. Published online June 28, 2023
-
DOI: https://doi.org/10.3803/EnM.2023.1688
-
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
This study evaluated the efficacy and safety of add-on gemigliptin in patients with type 2 diabetes mellitus (T2DM) who had inadequate glycemic control with metformin and dapagliflozin.
Methods
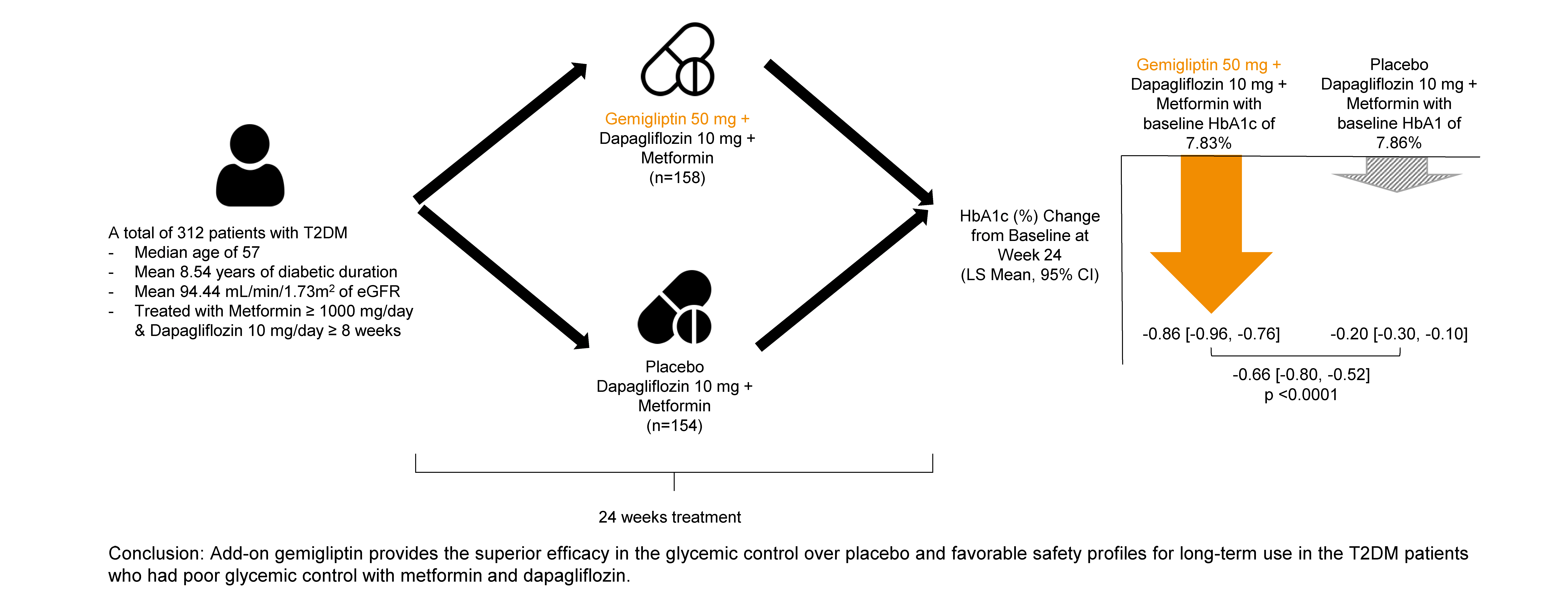
In this randomized, placebo-controlled, parallel-group, double-blind, phase III study, 315 patients were randomized to receive either gemigliptin 50 mg (n=159) or placebo (n=156) with metformin and dapagliflozin for 24 weeks. After the 24-week treatment, patients who received the placebo were switched to gemigliptin, and all patients were treated with gemigliptin for an additional 28 weeks.
Results
The baseline characteristics were similar between the two groups, except for body mass index. At week 24, the least squares mean difference (standard error) in hemoglobin A1c (HbA1c) changes was –0.66% (0.07) with a 95% confidence interval of –0.80% to –0.52%, demonstrating superior HbA1c reduction in the gemigliptin group. After week 24, the HbA1c level significantly decreased in the placebo group as gemigliptin was administered, whereas the efficacy of HbA1c reduction was maintained up to week 52 in the gemigliptin group. The safety profiles were similar: the incidence rates of treatment-emergent adverse events up to week 24 were 27.67% and 29.22% in the gemigliptin and placebo groups, respectively. The safety profiles after week 24 were similar to those up to week 24 in both groups, and no new safety findings, including hypoglycemia, were noted.
Conclusion
Add-on gemigliptin was well tolerated, providing comparable safety profiles and superior efficacy in glycemic control over placebo for long-term use in patients with T2DM who had poor glycemic control with metformin and dapagliflozin.
- Adrenal gland
Big Data Articles (National Health Insurance Service Database)
- Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
-
Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
-
Endocrinol Metab. 2023;38(2):253-259. Published online March 21, 2023
-
DOI: https://doi.org/10.3803/EnM.2022.1607
-
-
2,666
View
-
103
Download
-
2
Web of Science
-
2
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
The severity of coronavirus disease 2019 (COVID-19) among patients with long-term glucocorticoid treatment (LTGT) has not been established. We aimed to evaluate the association between LTGT and COVID-19 prognosis.
Methods
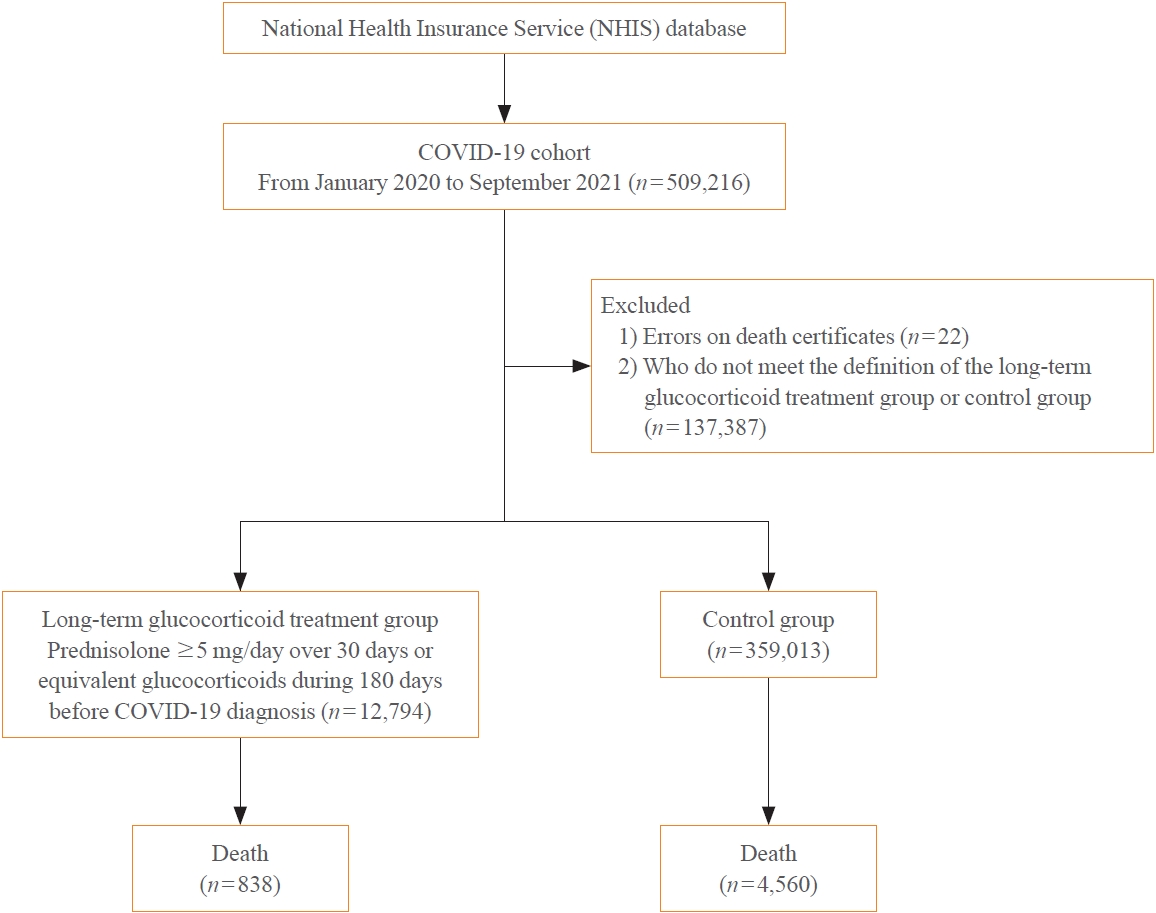
A Korean nationwide cohort database of COVID-19 patients between January 2019 and September 2021 was used. LTGT was defined as exposure to at least 150 mg of prednisolone (≥5 mg/day and ≥30 days) or equivalent glucocorticoids 180 days before COVID-19 infection. The outcome measurements were mortality, hospitalization, intensive care unit (ICU) admission, length of stay, and mechanical ventilation.
Results
Among confirmed patients with COVID-19, the LTGT group (n=12,794) was older and had a higher proportion of comorbidities than the control (n=359,013). The LTGT group showed higher in-hospital, 30-day, and 90-day mortality rates than the control (14.0% vs. 2.3%, 5.9% vs. 1.1%, and 9.9% vs. 1.8%, respectively; all P<0.001). Except for the hospitalization rate, the length of stay, ICU admission, and mechanical ventilation proportions were significantly higher in the LTGT group than in the control (all P<0.001). Overall mortality was higher in the LTGT group than in the control group, and the significance remained in the fully adjusted model (odds ratio [OR], 5.75; 95% confidence interval [CI], 5.31 to 6.23) (adjusted OR, 1.82; 95% CI, 1.67 to 2.00). The LTGT group showed a higher mortality rate than the control within the same comorbidity score category.
Conclusion
Long-term exposure to glucocorticoids increased the mortality and severity of COVID-19. Prevention and early proactive measures are inevitable in the high-risk LTGT group with many comorbidities.
-
Citations
Citations to this article as recorded by  - Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy
Eun-Hee Cho
Endocrinology and Metabolism.2023; 38(2): 223. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- Diabetes, obesity and metabolism
Big Data Articles (National Health Insurance Service Database)
- Risk for Newly Diagnosed Type 2 Diabetes Mellitus after COVID-19 among Korean Adults: A Nationwide Matched Cohort Study
-
Jong Han Choi, Kyoung Min Kim, Keeho Song, Gi Hyeon Seo
-
Endocrinol Metab. 2023;38(2):245-252. Published online April 5, 2023
-
DOI: https://doi.org/10.3803/EnM.2023.1662
-
-
2,319
View
-
122
Download
-
2
Web of Science
-
2
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
Coronavirus disease 2019 (COVID-19) can cause various extrapulmonary sequelae, including diabetes. However, it is unclear whether these effects persist 30 days after diagnosis. Hence, we investigated the incidence of newly diagnosed type 2 diabetes mellitus (T2DM) in the post-acute phase of COVID-19.
Methods
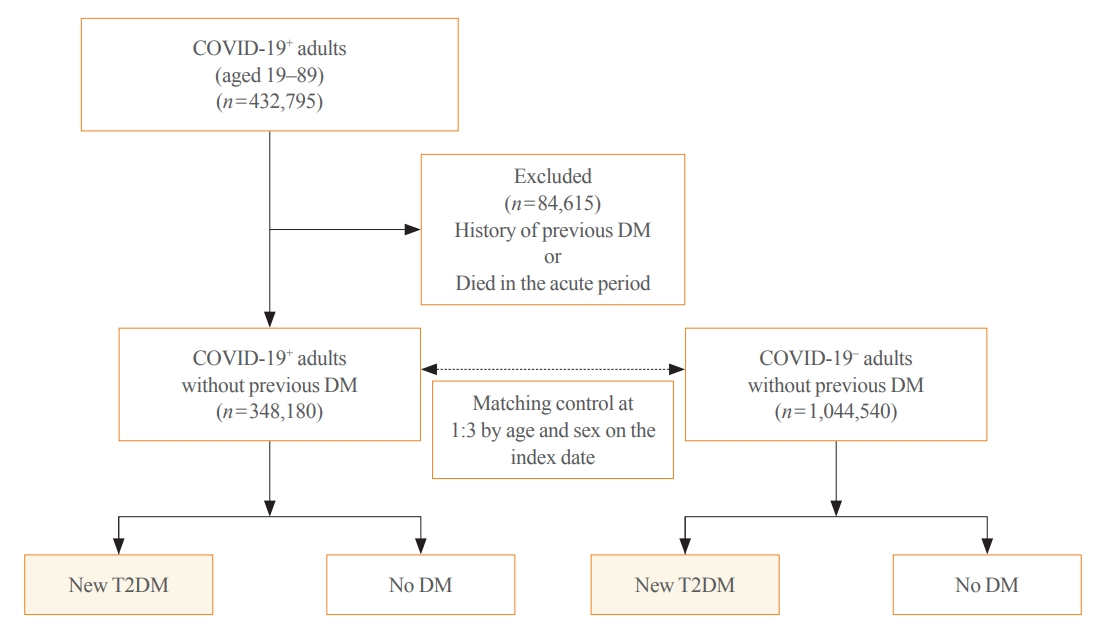
This cohort study used data from the Health Insurance Review and Assessment Service, a representative national healthcare database in Korea. We established a cohort of 348,180 individuals diagnosed with COVID-19 without a history of diabetes between January 2020 and September 2021. The control group consisted of sex- and age-matched individuals with neither a history of diabetes nor COVID-19. We assessed the hazard ratios (HR) of newly diagnosed T2DM patients with COVID-19 compared to controls, adjusted for age, sex, and the presence of hypertension and dyslipidemia.
Results
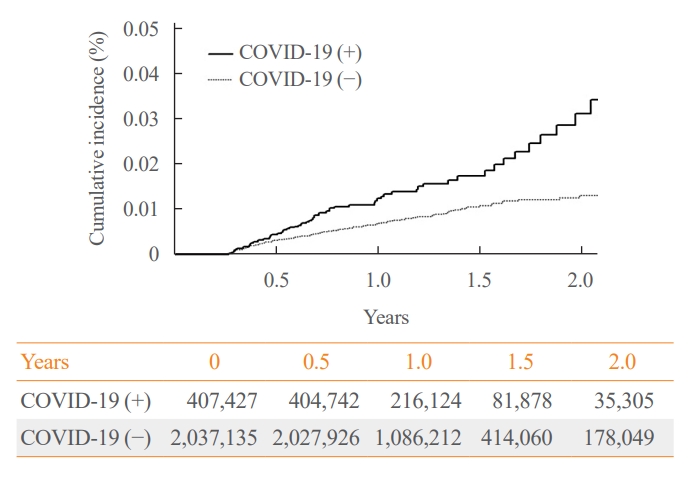
In the post-acute phase, patients with COVID-19 had an increased risk of newly diagnosed T2DM compared to those without COVID-19 (adjusted HR, 1.30; 95% confidence interval [CI], 1.27 to 1.33). The adjusted HRs of non-hospitalized, hospitalized, and intensive care unit-admitted patients were 1.14 (95% CI, 1.08 to 1.19), 1.34 (95% CI, 1.30 to 1.38), and 1.78 (95% CI, 1.59 to 1.99), respectively. The risk of T2DM in patients who were not administered glucocorticoids also increased (adjusted HR, 1.29; 95% CI, 1.25 to 1.32).
Conclusion
COVID-19 may increase the risk of developing T2DM beyond the acute period. The higher the severity of COVID-19 in the acute phase, the higher the risk of newly diagnosed T2DM. Therefore, T2DM should be included as a component of managing long-term COVID-19.
-
Citations
Citations to this article as recorded by  - New-Onset Diabetes Mellitus in COVID-19: A Scoping Review
Anca Pantea Stoian, Ioana-Cristina Bica, Teodor Salmen, Wael Al Mahmeed, Khalid Al-Rasadi, Kamila Al-Alawi, Maciej Banach, Yajnavalka Banerjee, Antonio Ceriello, Mustafa Cesur, Francesco Cosentino, Alberto Firenze, Massimo Galia, Su-Yen Goh, Andrej Janez,
Diabetes Therapy.2024; 15(1): 33. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
|